Serial Dilution Advantages And Disadvantages
Muddy heights full screen. It got down to the wire but Muddy Heights is now finished! Check it out at: gamejolt.com/games/arcade/muddy-heights/39301. Muddy Heights® 2 Free Download PC Game Cracked in Direct Link and Torrent. Muddy Heights® 2 – Poop you way to victoryagain! In Muddy Heights 2, you play as a person who has had a little bit too much to eat and needs to relieve himself by any means. TORRENT – FREE DOWNLOAD – CRACKED. Muddy Heights® 2 – Poop you way to victoryagain! In Muddy Heights 2, you play as a person who has had a little bit too much to eat and needs to relieve himself by any means.
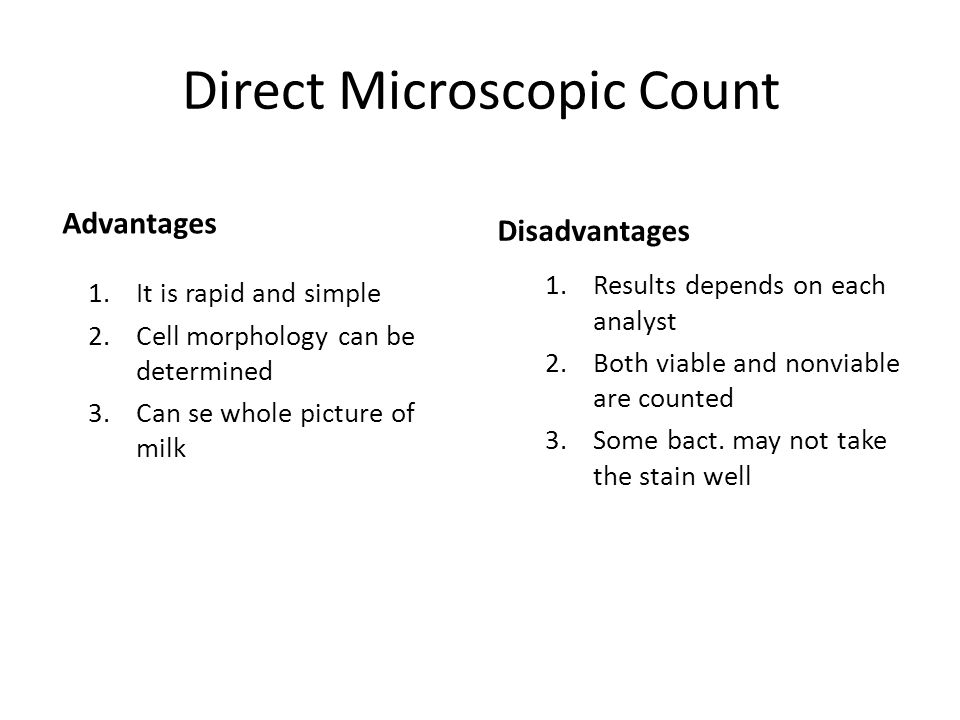
Answer to Whats the advantages and disadvantages for each of the following: 1) Preparing solutions by serial dilution. The serial dilution technique1 using pipets is a well-established way to. Direct dilution provides a number of advantages over serial dilution. • This technique is based upon the ability of a single organism to multiply in the. What is the advantages and disadvantages to the serial dilution agar plate.
Advantages of 'Serial Dilutions' This section is not a recipe for your experiment. It explains some principles for designing dilutions that give optimal results. Once you understand these principles, you will be better able to design the dilutions you need for each specific case. Often in experimental work, you need to cover a range of concentrations, so you need to make a bunch of different dilutions.
For example, you need to do such dilutions of the standard IgG to make the standard curve in ELISA, and then again for the unknown samples in ELISA. You might think it would be good to dilute 1/2, 1/3, 1/10, 1/100. These seem like nice numbers. There are two problems with this series of dilutions. • The dilutions are unnecessarily complicated to make. You need to do a different calculation, and measure different volumes, for each one. It takes a long time, and it is too easy to make a mistake.
• The dilutions cover the range from 1/2 to 1/100 unevenly. In fact, the 1/2 vs. 1/3 dilutions differ by only 1.5-fold in concentration, while the 1/10 vs. 1/100 dilutions differ by ten-fold.
If you are going to measure results for four dilutions, it is a waste of time and materials to make two of them almost the same. And what if the half-maximal signal occurs between 1/10 and 1/100? You won't be able to tell exactly where it is because of the big space between those two. Serial dilutions are much easier to make and they cover the range evenly. Serial dilutions are made by making the same dilution step over and over, using the previous dilution as the input to the next dilution in each step. Since the dilution-fold is the same in each step, the dilutions are a geometric series (constant ratio between any adjacent dilutions).
We wanted to make a narrative-style Paragliding video without using a single GoPro shot that focused on the philosophy of a true pilot. I met Pascal long before I knew he wanted to do the X-Alps and we started the video before he was selected to participate. Pasport gazovoj pliti elekta form.
For example: 1/3, 1/9, 1/27, 1/81 Notice that each dilution is three-fold relative to the previous one. In four dilutions, we have covered a range of 181/3 = 60-fold. If that isn't enough range, consider a series of five-fold dilutions: 1/5, 1/25, 1/125, 1/625 Here we've covered a (625/5) = 125-fold range. No matter where the half-max falls in a series of 5-fold dilutions, it is no more than 2.2-fold ('middle' [square root] of a 5-fold step) away from a data point -- so the coverage of the range is thorough and even. When you need to cover several factors of ten (several 'orders of magnitude') with a series of dilutions, it usually makes the most sense to plot the dilutions (relative concentrations) on a logarithmic scale. This avoids bunching most of the points up at one end and having just the last point way far down the scale.